Part 2: The Ins and Outs of Prescribing Buprenorphine

A Review
In part one of this blog post, we introduced the case of Mr. D, a 54-year-old male with stable Stage IV lung cancer, under active treatment with immunotherapy. With a history of chronic pleuritic pain, and long-term usage of a fentanyl patch and oxycodone (200 mg morphine equivalents daily) for pain management, Mr. D developed opioid misuse behaviors, and met DSM-5 criteria for opioid use disorder (OUD). After a shared-decision, Mr. D’s palliative care team rotated him to 4 mg buprenorphine/1 mg naloxone treatment (three times/day), which allowed him to achieve pain control, while treating his OUD. He has done well on this regimen, and not only have his opioid misuse behaviors abated, but his quality of life and pain have improved.
In part two, we discuss when palliative care clinicians should consider introducing buprenorphine for their patients living with serious illness. We also explore barriers to use, including access and cost, and a tip that may help with insurance coverage for patients.
When to Consider Prescribing Buprenorphine for People Living with Serious Illness
Given buprenorphine’s safety profile and the pharmacology described in part one, palliative care clinicians may find it useful when treating patients living with serious illness with a variety of pain and opioid challenges. Possible buprenorphine indications in people living with serious illness are outlined in Figure 1, below. After that, we expand upon the use of buprenorphine for pain, enhanced safety, and OUD, and prescribing considerations.
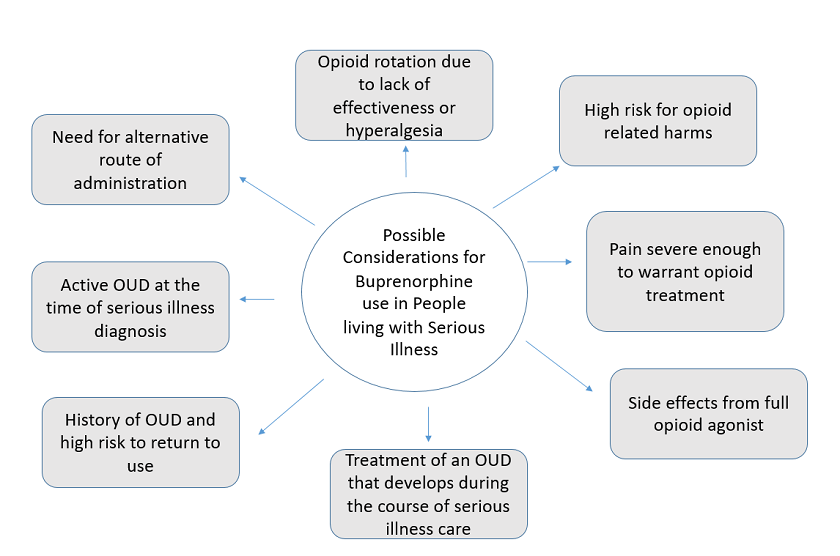
1. Buprenorphine for pain
In many situations, such as serious illness without OUD, buprenorphine may have a comparable analgesic effect to full µ-opioid receptor agonists for pain. Expert consensus recommends that buprenorphine’s classification as a partial µ-opioid receptor agonist not be clinically translated to mean partial analgesic efficacy. While historically buprenorphine has not been considered first-line opioid therapy, it may be a good initial option for patients with an increased risk of harm with a full opioid agonist. This may include individuals with a history of opioid misuse, OUD, or other substance use disorder; uncontrolled psychiatric disorder who are at higher risk of experiencing opioid misuse or OUD; or older adults with a higher risk of experiencing opioid side effects (e.g., constipation, delirium, or respiratory depression).
2. Buprenorphine rotation to reduce risks and harms in individuals on long-term opioid therapy
In light of the mounting evidence of harms of long-term full-agonist opioid therapy, including decreased function/return to work, increased rates of depression, motor vehicle accidents, falls, endocrine dysfunction, overdose, and OUD, clinicians taking care of patients with serious illness must invoke a benefit to risk/harm framework for all opioid decisions. This includes reconsidering long-term opioid therapy in patients who have a serious illness but are expected to have a longer (e.g., > 1 year) prognosis. This especially applies to people at high risk for opioid harm, such as those requiring high opioid doses, co-prescribed other sedating medications, or who have comorbidities.
Rotation to buprenorphine has the potential to reduce opioid-related risks as described above. It can also reduce the risk of hyperalgesia, which is a state of heightened sensitivity to pain, caused by exposure to opioids. While hyperalgesia is understudied, rotation to buprenorphine is an approach that has been used in practice and, at times, results in improved pain and opioid side effects.
The use of buprenorphine to facilitate patient-centered opioid tapering is a strategy endorsed by the Health and Human Service Guidelines (HHS) for Discontinuation of Long-Term Opioid Analgesics. In the context of opioid discontinuation, a shared individualized discussion should occur between patient and clinician, grounded on the individual benefits and harms of opioid treatment. For some, there may be challenges to tolerating the opioid taper—buprenorphine could be considered to manage pain and affective symptoms during the opioid taper. This approach has not been empirically supported but is frequently reported as a strategy for patients with protracted abstinence syndrome or complex persistent opioid dependence.
3. Buprenorphine for OUD
Some people who are diagnosed with a serious illness have pre-existing OUD. Others may experience pain as a result of their serious illness, are prescribed opioids, and over a period of time, develop symptoms and behaviors that may be concerning, leading to an OUD diagnosis. Importantly, serious illness does not preclude patients from developing an opioid use disorder.
While a diagnosis of OUD may sometimes be clear-cut, this is not always the case. Palliative care clinicians should be prepared to safely and effectively diagnose and treat patients with serious illness—who are also suffering with OUD. Buprenorphine offers an option that can be used for patients with high-risk, misuse, or suspected or active OUD—without delaying treatment for days, weeks, or months while trying to establish a diagnosis of OUD.
Common Buprenorphine Formulations for Pain and OUD
| Buprenorphine for pain | Buprenorphine for OUD | |
|---|---|---|
| DEA X waiver required | NO | No longer required due to the Omnibus bill |
| FDA approved formulation |
Buprenorphine buccal film (Belbuca) Buprenorphine transdermal patch (Butrans) Buprenorphine injectable solution (Buprenex) |
Buprenorphine/naloxone (Suboxone) |
| Dosing considerations |
Buprenorphine buccal film (Belbuca) - Route: buccal - Formulation: film - Doses range from 75 mcg-900 mcg - Doses once/day or twice/day Buprenorphine transdermal patch (Butrans) - Route: transdermal - Dosed every 7 days - Doses range 5 mcg-20 mcg/hr Buprenorphine injectable solution (Buprenex) - Route: intravenous or intramuscular - Dosed every q6hr as needed - Typical dose is 0.3 mg |
Buprenorphine/naloxone (Suboxone) - Buprenorphine/naloxone combination product - Route: sublingual or buccal - Available ranges from: 2 mg buprenorphine/0.5 naloxone to 12mg buprenorphine/3 mg naloxone - Doses for OUD are typically 12-24 mg of buprenorphine once a day (max dose 32 mg/day) - Buprenorphine/naloxone combination product is the most common formulation used off-label for chronic pain and opioid tapering often dosed at smaller doses (4-12 mg/day) BID to TID for pain - The combination product of buprenorphine with naloxone was developed to decrease misuse and diversion. If buprenorphine/naloxone product is taken as prescribed (sublingually) the naloxone portion is not active. However, if the combination product is injected the naloxone portion becomes active and may result in opioid withdrawal. Formulations less used in patients with serious illness include buprenorphine alone without naloxone (brand name Subutex) and extended release injectable buprenorphine (brand name Sublocade). |
Barriers to Buprenorphine Use For Both Pain and OUD
Since the passage of the Affordable Care Act, sublingual buprenorphine/naloxone for OUD is consistently paid for by insurers. This means if you diagnose OUD and treat comorbid pain and OUD with buprenorphine/naloxone, it will be covered.
For OUD treatment, barriers to buprenorphine use mainly center around stigma, which is entrenched in the health care system. This may include pharmacy barriers, such as an unwillingness to fill prescriptions or limited access to subacute rehabilitation and long-term care facilities because of buprenorphine treatment.
Critical to overcoming barriers is to address stigma by treating OUD and advocating for buprenorphine insurance coverage when it is clinically indicated. In our experience, many barriers to buprenorphine use for pain and OUD are malleable by building relationships with interdisciplinary colleagues, advocating for patients, and providing education on the effectiveness and unique properties of buprenorphine.
For pain, some of the barriers may be more difficult, including payment and access issues. Unfortunately, certain formulations of buprenorphine may not covered by some commercial insurers or may be associated with high co-pays because other pain-modifying treatments are far less expensive. If a person living with serious illness does experience opioid side effects with a full opioid agonist or use a full agonist is ill-advised, it is recommended that you complete a prior authorization or appeal to the specific insurer.
Conclusion
Buprenorphine is a highly versatile opioid, with many benefits for patients living with serious illness. While its complex receptor pharmacology, regulatory issues, and various formulations may create hesitation in some palliative care clinicians, buprenorphine’s safety and efficacy should cause us to reconsider. We encourage all clinicians treating patients with serious illness to become familiar with prescribing buprenorphine and adding buprenorphine to their toolbox.
This Learning Pathway contains eight hours of AACME and ANCC accredited training in the management of opioid and substance use disorders required for all DEA-registered clinicians by The Medication Access and Training Expansion (MATE) Act. Visit the DEA Diversion Control website for complete instructions and an overview of this new requirement. Upon completion, learners will receive a downloadable certificate.
Get StartedEdited by Melissa Baron. Clinical review by Andrew Esch, MD, MBA.
*This blog post was updated on 3/14/2024 to remove references to the previous X-Waiver requirement.

